For thirty years, scientists have been asking the same question: why do tumors with hereditary BRCA2 mutations eventually stop responding to chemotherapy?
Research from Arnab Ray Chaudhuri’s lab (Erasmus MC) in collaboration with Raviprasad Kuthethur from the lab of Oncode Investigator Roland Kanaar (Erasmus MC) - with support from Oncode Institute in funding, infrastructure, and expertise in protein structure modeling - has now provided the answer. The researchers discovered how BRCA2-deficient tumors manage to repair DNA damage and survive treatment, and how that resistance might be reversed.
The findings, now published in Science, reshape what we know about BRCA2 and open new possibilities for tackling chemoresistant cancers.
Understanding Chemoresistance
One of the toughest problems in cancer treatment is chemoresistance: tumors that initially respond well to chemotherapy later stop reacting. Once that happens, options are limited.
The collaborative study has uncovered how this resistance develops in BRCA2-mutated tumors. Their work shows that the proteins BRCA2 and FIGNL1 play more complex roles in DNA repair than scientists once thought.
“These findings change the paradigm of thought,” says Ray Chaudhuri. “We identified not only how resistance arises, but also potential ways to prevent or reverse it.”
The study’s experiments were mainly driven by Oncode Researcher Raviprasad Kuthethur, first author of the publication.
An Unexpected Discovery
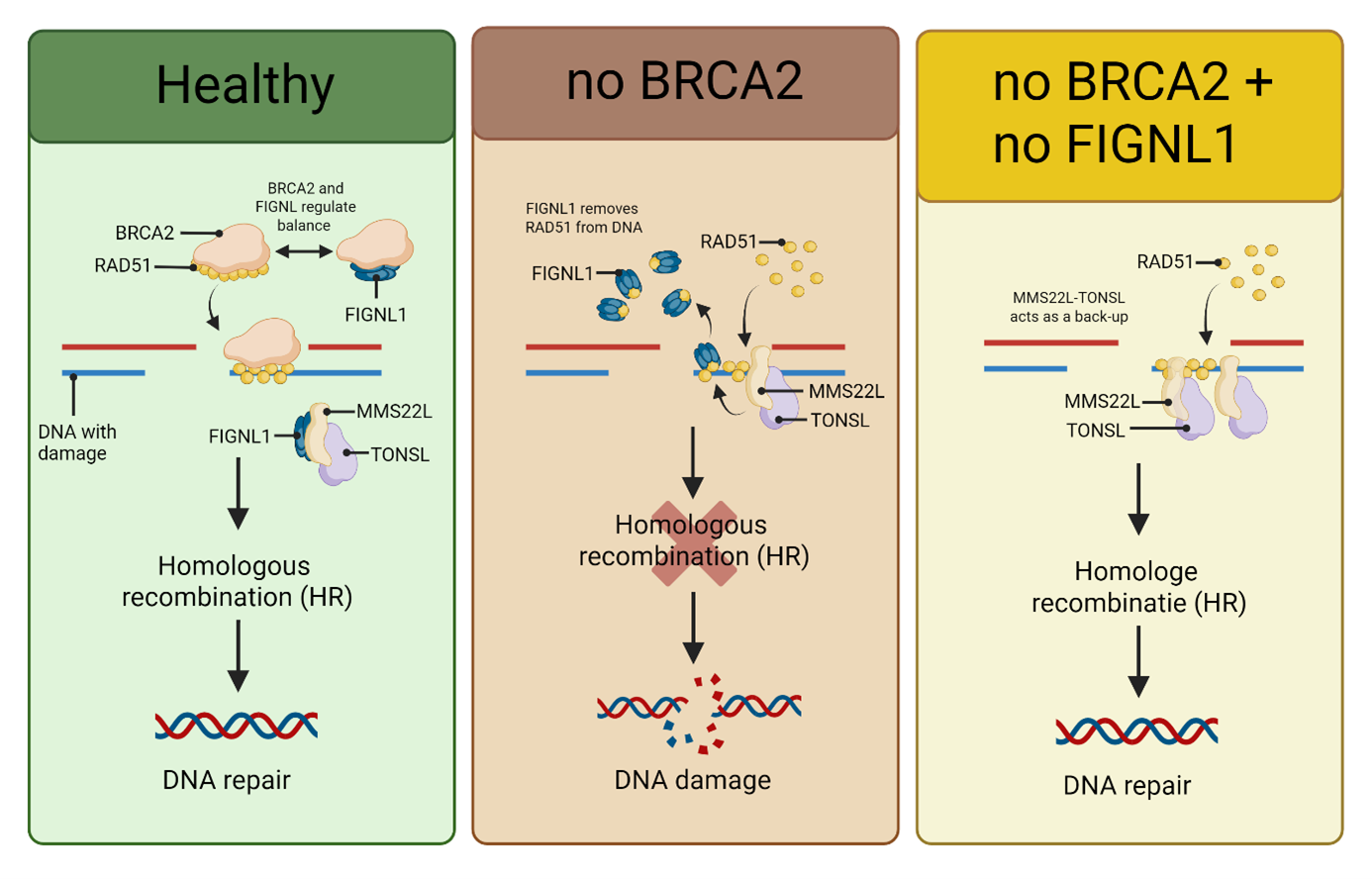
In cells missing BRCA2, the researchers made a surprising observation: when they removed another protein, FIGNL1, the cells suddenly regained their ability to repair DNA through homologous recombination (HR) - the main pathway cells use to fix broken DNA strands.
“The result was completely unexpected,” says Kuthethur. “It took a long time before we truly believed what we were seeing.”
Further experiments revealed how this happens. FIGNL1 normally removes another protein, RAD51, from damaged DNA. Without RAD51, HR can’t take place. But when FIGNL1 is disabled, RAD51 stays attached, allowing DNA repair to continue even without BRCA2.
BRCA2 as a Regulator
For decades, BRCA2 was believed to be the key player in loading RAD51 onto damaged DNA. This new work challenges that view.
“In healthy cells, BRCA2 and FIGNL1 actually work together,” explains Ray Chaudhuri. “BRCA2 helps RAD51 bind to DNA, while FIGNL1 removes it. Together, they keep the process in balance.”
That balance ensures DNA damage is repaired efficiently - not too little, not too much.
A Backup System
When both BRCA2 and FIGNL1 are missing, the cell doesn’t give up. Instead, it switches to a backup system involving another protein complex called MMS22L–TONSL.
This complex can also help RAD51 attach to DNA, keeping repair going — and, unfortunately, allowing cancer cells to survive chemotherapy.
“If we block MMS22L–TONSL, the entire mechanism collapses,” says Ray Chaudhuri. “That could make resistant BRCA2-mutated tumors sensitive to chemotherapy again.”
Impact on Science and Health
This research fundamentally changes how scientists view BRCA2’s role in DNA repair. By revealing how BRCA2, FIGNL1, and MMS22L–TONSL interact, it explains how BRCA2-deficient tumors can regain DNA repair ability and develop resistance to chemotherapy.
The discovery points to a new therapeutic angle: targeting the MMS22L–TONSL pathway to make resistant tumors vulnerable again.
The work was supported by Oncode Institute, which provided funding, infrastructure, and expertise in structural biology through AlphaFold-based protein modelling. This multidisciplinary support was essential in uncovering the molecular interactions that drive the repair mechanism.
Beyond BRCA2, the study also highlights how cancer cells adapt - finding alternative routes to survive treatment. Understanding these molecular “workarounds” could help researchers design smarter, more durable therapies for patients.