Neatly rolled up in a cell nucleus of only a hundredth of a millimetre in size, our genome consists of approximately two metres of DNA spread over 46 chromosomes. Three Oncode Investigators explain why they are fascinated by the spatial organization of this tiny ball of thread.
A complete package of human genetic material (the genome) contains approximately 22,000 genes. The activity of these genes (gene expression) is regulated by millions of switches that lie scattered over the chromosomes. Oncode Investigator dr. Wouter de Laat of the Hubrecht Institute explains: “The appropriate regulation of gene expression constitutes the basis of a healthy functioning cell. Many tumour cells display a profound disturbance of this delicate balance, with genes being either too active or too silent. As gene expression is affected by the spatial organization of the chromosomes, cancer researchers all over the world are studying the three-dimensional organization of the genome.”
Hair balls
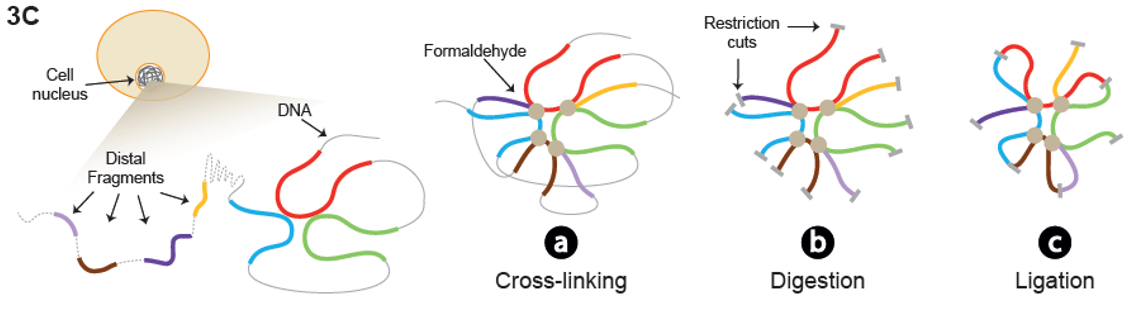
De Laat shares an example from his own research group: “Together with Ruud Delwel of the Erasmus MC, we have studied about 40 patients with acute myeloid leukaemia. These people all had an inversion on chromosome 3; this is a genomic aberration in which a DNA fragment is flipped 180 degrees. We studied the spatial organization of the patients’ chromosomes using the so-called ‘4C technique’. Here, researchers fixate chromosomes with a chemical (formaldehyde), cross-linking chromosomal fragments that are adjacent in the cell. Then, they cut the cross-linked DNA with specialized proteins (restriction enzymes), resulting in hairball-like structures. By analysing the hairballs’ composition, we can deduce which chromosomal fragments are spatially close in the cell.”
“In the leukaemia patients, we discovered that the inversion altered the spatial position of a DNA fragment that enhances gene activity. While this enhancer is normally located in the proximity of a tumour suppressor gene, the inversion moved it to the proximity of a cancer-causing gene (an oncogene). Hence, this spatial reorganization had a double disastrous consequence. Indeed, these patients suffered from a highly aggressive form of leukaemia. Delwel’s group is now exploring how this insight may be translated in therapeutic options,” says de Laat.
Ink pads
De Laat’s colleague at the Hubrecht Institute, dr. Jop Kind, also studies the three-dimensional organization of the genome: “I am interested in the position of chromosomes within the cell nucleus. Active sections of chromosomes are usually located in the centre of the nucleus, whereas inactive parts are near the edge. This spatial architecture alters as a healthy cell transforms into a tumour cell. We use the ‘DamID’ technique to study this process. Here, we fuse a protein called ‘Dam’ to the nucleus’ inner wall. Dam acts as an ink pad, adding a methyl group to each A in the DNA. We then extract the DNA from the nucleus and analyse which chromosomal fragments contain methylated A’s, inferring which parts have touched the inner wall of the nucleus. This is as much as 30-40% of the genome. We can do this for individual cells at various stages of tumour progression.” (Watch this video to see how DamID works.)

Kind applies the DamID technique to ingenious structures developed by his colleagues at the Hubrecht Institute. “The group of Hans Clevers have developed miniature artificial colons to study tumour progression. In these organoids, they have introduced a series of four oncogenic mutations stepwise. Starting with healthy mini colons, they have first mutated the APC gene, then P53, KRAS, and finally SMAD4. These quadruple-mutated mini colons behave as a metastasising tumour when transplanted into a mouse. We use DamID to visualize the changes in the three-dimensional position of the chromosomes along the pathway from healthy to metastatic tumour cell. We hope to discover why some cells metastasize, while the majority do not. Understanding this in detail may enable scientists to develop a method to predict or even prevent metastasis in the future.”
Folds
Meanwhile at the Netherlands Cancer Institute, Oncode Investigator dr. Elzo de Wit is involved in an experiment where researchers actively alter chromosomal folding within the nuclei of laboratory cells. He explains: “We have silenced the so-called ‘Wapl’ protein in mouse embryonic stem cells. As a result, the chromosomes gradually alter their folding, creating more loops. We investigate how this affects gene expression and cell differentiation. Interestingly, some parts of the genome are much more sensitive to Wapl depletion than others. We want to understand what this means in health and disease.”
De Wit has a computational background. “I find it highly exciting to extract meaningful relationships from the massive haystacks of research data that we generate. In addition, it is a challenge to develop smart tools that can assist researchers in analysing their data.” Kind adds: “For me, curiosity is the main motivation. Cells fascinate me: they are such ingenious systems. The desire to thoroughly understand how the cell switches its genes on and off and what happens when this mechanism is disturbed, drives me to the lab every day.”
Application
“I feel privileged that I have been able to bring my work to the clinic,” replies de Laat upon asking about his personal drive. He is referring to an unexpected clinical application of the 4C technique, in which de Wit was also involved. The researchers discovered that the technique is highly suited to diagnose genomic translocations, genetic aberrations in which genetic material is exchanged between chromosomes. De Laat: “Translocations are also frequently found in tumours and 4C is uniquely suited for diagnostics on paraffin-embedded pathology samples.”
In 2012, De Laat and de Wit co-founded biotech company ‘Cergentis’ to bring 4C and a related diagnostic technology (Targeted Locus Amplification) to the clinic. Cergentis currently employs ~20 people, including several of de Laat’s former PhD students and technicians. De Laat: “Nobody could have imagined that our fundamental research on DNA folding would result in a biotech company that offers improved genetic tests to hospitals and the pharmaceutical industry. That is exactly the reason why I am a passionate advocate of fundamental research. True innovation in cancer treatment relies on new insights into the basic mechanisms of cancer development. That is also why Oncode Institute focuses on molecular oncology. Oncode enables scientists to pursue challenging research projects that might fail … but will truly impact patients’ lives if they succeed.”
Future
“In the coming years, we will learn in great detail about the spatial regulatory contacts of our genome. There is no doubt this will lead to new insights into the causes of cancer. This will help clinicians stratify patients and it will guide the search for targeted therapeutic interventions. In parallel, dedicated efforts are ongoing to bring these novel methodologies for improved diagnostics to the clinic,” concludes de Laat.
Wouter de Laat is one of the authors of a recent publication in Nature Genetics. It describes a new methodology to study genome conformation called ‘Multi-Contact Chromatic Conformation Capture’. Read more here.