HTLF was already known to be involved in the DNA damage response as an important protein for replicating cells in the post replication repair pathway. But new research from the lab of Oncode Investigator Jurgen Marteijn (Erasmus MC) now revealed a completely different key function of it. The scientists uncovered that the RAD5 related translocase HLTF plays a crucial role in Nucleotide Excision Repair (NER) for the active eviction of the damaged DNA. The findings of this study have been published in Molecular Cell today.
Nucleotide excision repair (NER) recognizes a wide variety of DNA damage, including DNA damage induced by UV-light of the sun, or chemotherapy. By doing so, it protects against the onset of cancer. Some families have inborn mutations in this pathway, and they develop xeroderma pigmentosum, a hereditary condition characterized by extreme sun sensitivity. They have a 1000 times higher chance than others of developing skin cancer in the parts of their bodies that get exposed to the sun. And there’s no treatment for this. This set of patients is important for this research. They give good insight into how this highly relevant repair mechanism works. Thinking of cancer – NER is highly important for genome stability, on one end counteracting the onset of cancer and it is often found mutated in cancer cells, but it’s also one of the repair pathways that is involved in resolving DNA damage-induced by chemotherapy common anti-cancer drugs like Cisplatin.
After damage recognition by the NER pathway occurs, the damaged DNA is cut out by nucleases. The regulatory mechanisms that drive NER through its successive damage recognition, verification, incision and gap restoration reaction steps remained thus far largely elusive. In this new study, Marteijn and his team have identified that HLTF, a protein commonly mutated in cancers, is responsible for the active eviction of the incised, but not yet removed, damaged DNA together with associated repair proteins. This represents an additional quality control step by coordinating the transition between the excision and DNA synthesis steps to safeguard genome integrity.
The project started 6 years ago. Two PhD students in Marteijn’s lab were involved: Yasemin Turkyilmaz who worked on identifying the factor and Marvin van Toorn who helped ‘nailing it down’ by understanding what it was doing. “Such a project takes time, but it really pays off”, says Marteijn. “It is for this kind of discovery that I do research, finding a new repair factor that is involved in a previously unknown reaction step. Maybe with some follow up support, it could be brought to something useful in the clinic.”
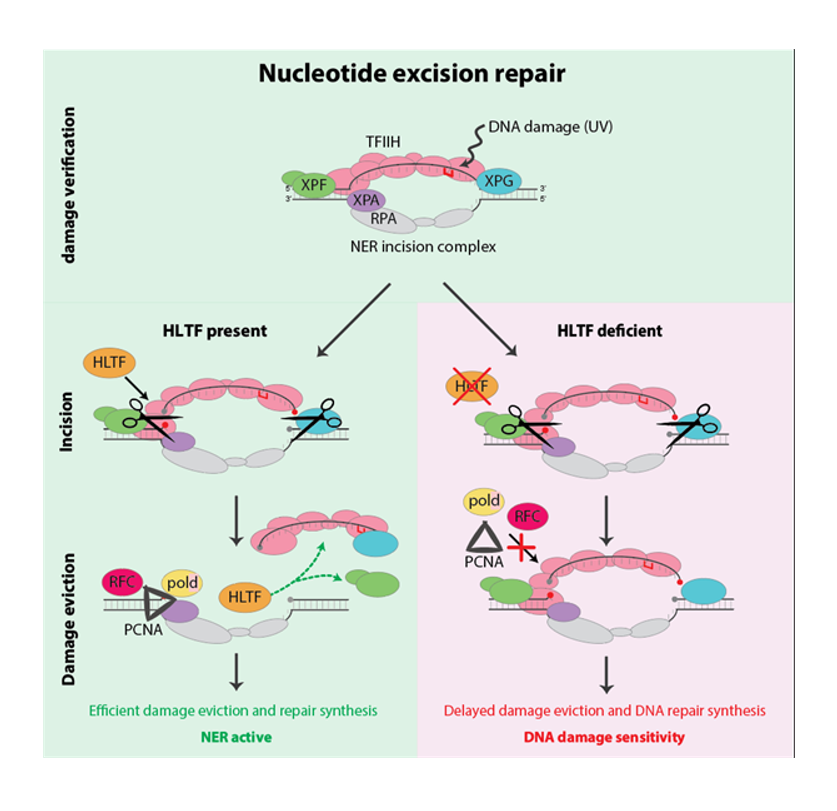
Looking towards therapeutic implications and possible next steps - HLTF is mutated or downregulated in many cancers. “A possible follow up at this point might be to develop a kind of personalized treatment for patients with cancers that are HLTF deficient. If we treat those patients with chemotherapeutics that are normally repaired by NER, that could have an extra beneficial effect, because those cancer cells cannot repair those therapy-induced DNA damages well, so they would become extra sensitive to those chemotherapies” concludes Marteijn.